Main page (Russian)
 Search in database (English)
Search in database (English)
Properties of substance:
3-(4-methoxyphenyl)propionic acid
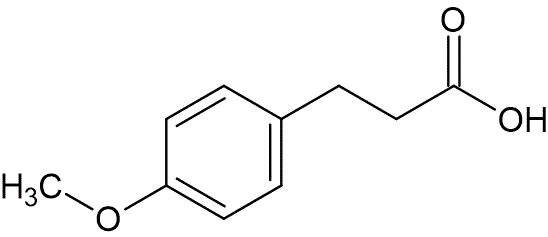
skc-fileSynonyms:
4-methoxydihydrocinnamic acid
Group of substances:
organic
Physical appearance:
crystalsEmpirical formula (Hill's system for organic substances):
C10H12O3Structural formula as text:
CH3OC6H4CH2CH2COOHMolar/atomic mass: 180.20048
Melting point (°C):
104-105CAS ¹: 1929-29-9
Synthesis 1:
Reference: Heffner, R. J., & Joullie, M. M. Synthetic Routes to Ninhydrins. Preparation of Ninhydrin, 5-Methoxyninhydrin, and 5-(Methylthio)Ninhydrin / Synthetic Communications. - 1991. - Vol. 21, No. 21 pp. 2241-2242 [doi: 10.1080/00397919108055457]
CH3OC6H4CH=CHCOOH + H2 → CH3OC6H4CH2CH2COOH
To a solution of 4-methoxycinnamic acid (9.3 g, 52.9 mmol) in methanol (340 mL), and acetic acid (58 mL), at 25 °C, was added 5% by weight of 10% palladium on activated carbon. The mixture was hydrogenated at a pressure of 45 psi. After one hour, the mixture was filtered through Celite to remove the catalyst. The solids were washed with methanol (50 mL). The filtrate was evaporated under reduced pressure, and the resulting residue azeotroped with toluene to remove residual acetic acid to give crude product (10 g, 95% yield). This solid was recrystallized from 2-propanol and water to afford pure 4-methoxydihydrocinnamic acid, mp 102.5—104 °C, lit. mp 103.5—104 °C.
Rf 0.55 methylene chloride : methanol : acetic acid (90 : 10 : 1).
IR (CHCl3): 3450-2400 (broad), 1712 (strong), 1612 (medium), 1585 (weak), 1510 (medium), 1465 (medium), 1455 (medium), 1442 (medium), 1412 (medium), 1302 (medium), 1245 (strong), 1180 (medium), 1130 (weak), 1107 (weak), 1036 (medium), 940 (weak), 830 (medium) cm-1.
1H NMR (CDCl3): δ 2.65 (2H, m), 2.91 (2H, m), 3.79 (3H, s), 6.83 (2H, m), 7.13 (2H, m).
Synthesis 2:
Reference: Heffner, R. J., & Joullie, M. M. Synthetic Routes to Ninhydrins. Preparation of Ninhydrin, 5-Methoxyninhydrin, and 5-(Methylthio)Ninhydrin / Synthetic Communications. - 1991. - Vol. 21, No. 21 pp. 2248 [doi: 10.1080/00397919108055457]
CH3OC6H4CH2CH2COOCH3 + KOH → CH3OC6H4CH2CH2COOK + CH3OH
CH3OC6H4CH2CH2COOK + HCl → CH3OC6H4CH2CH2COOH + KCl
To a solution of methyl 4-methoxydihydrocinnamate (7.50 g, 38.6 mmol) in methanol (125 mL) and water (10 mL), at 25 °C, was added potassium hydroxide (6.5 g, 0.12 mol). After 2 hours, the reaction mixture was evaporated under reduced pressure to a solid residue, which was dissolved in water (100 mL), and acidified with 12N hydrochloric acid at 5 °C. The solid that precipitated upon addition of the acid was filtered, washed with water, and dried over P2O5 to afford 4-methoxydihydrocinnamic acid (6.94 g, 94% yield). This compound was recrystallized from 2-propanol and water to afford the pure acid.Synthesis 3:
Reference: Synthetic Communications. - 1991. - Vol. 21, No. 21 pp. 2248-2249 [doi: 10.1080/00397919108055457]To a solution of 4-methoxycinnamic acid (20.0 g, 0.112 mol) in methanol (500 mL), at 20 °C, was added magnesium metal turnings (10.9 g, 0.449 mol). [CAUTION: Redistilled, dry methanol must be used, and the temperature of the reaction mixture must be controlled carefully as this reaction is extremely exothermic.] After a steady evolution of hydrogen gas was initiated, the reaction mixture was cooled to 10 °C. After 1.5 hours the reaction was poured into a 12N HCl-ice mixture (200 mL), and extracted three times with ethyl acetate (200 mL). The organic extracts were combined and concentrated under reduced pressure, then diluted with ethyl acetate (300 mL). The organic layer was washed with saturated sodium chloride solution, dried over Na2SO4, and concentrated under reduced pressure to afford 4-methoxydihydrocinnamic acid (20 g, 99% yield). This solid was recrystallized from 2-propanol and water to afford pure 4-methoxydihydrocinnamic acid.
Dissociation:
pKa (1) = 4.69 (25°C, water)
References:
- Dictionary of organic compounds. - Vol. 2, Eccaine - Myrtillin chloride. - London, 1946. - pp. 601
- Ñïðàâî÷íèê õèìèêà. - Ò. 3. - Ì.-Ë.: Õèìèÿ, 1965. - pp. 91 [Russian]
Your requests if no data into database
© Collected Ruslan Anatolievich Kiper, burewestnik@mail.ru

