Main page (Russian)
 Search in database (English)
Search in database (English)
Properties of substance:
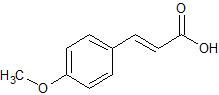
p-methoxycinnamic acid
skc-fileGroup of substances:
organic
Physical appearance:
needles crystalsEmpirical formula (Hill's system for organic substances):
C10H10O3Structural formula as text:
CH3OC6H4CH=CHCOOHMolar/atomic mass: 178.185
Melting point (°C):
175CAS №: 943-89-5
Solubility (g/100 g of solvent):
acetic acid: soluble [Ref.]
carbon tetrachloride: soluble [Ref.]
ethanol: sparingly soluble [Ref.]
water: 0.00712 (20°C) [Ref.]
Synthesis 1:
Reference: Heffner, R. J., & Joullie, M. M. Synthetic Routes to Ninhydrins. Preparation of Ninhydrin, 5-Methoxyninhydrin, and 5-(Methylthio)Ninhydrin / Synthetic Communications. - 1991. - Vol. 21, No. 21 pp. 2241 [doi: 10.1080/00397919108055457]
CH3OC6H4CHO + CH2(COOH)2 → CH3OC6H4CH=CHCOOH + H2O + CO2
A solution of p-anisaldehyde (10 g, 73.4 mmol), malonic acid (11.5 g, 110 mmol), pyridine (13.6 mL, 168 mmol), and piperidine (0.5 mL) was heated to 110 °C. After heating for 3 hours, the reaction was cooled in an ice bath, diluted with ethyl ether (100 mL), and acidified with 2N HCl (84 mL). The resulting precipitate was filtered and the solid rinsed with ethyl ether to give 4-methoxycinnamic acid (11.3 g, 87% yield) as a white solid, which was further purified by recrystallization from 2-propanol, mp 173—175 °C, lit. mp 171.6—173.2 °C.
Rf 0.65 methylene chloride : methanol (90 : 10).
HRMS calcd. for C10H10O3 (M+): 178.063. Found: 178.061.
IR (KBr): 3360-2000 (broad), 1682 (strong), 1625 (medium), 1600 (strong), 1501 (medium), 1460 (weak), 1445 (medium), 1430 (medium), 1415 (medium), 1320 (strong), 1290 (weak), 1258 (strong), 1220 (strong), 1193 (medium), 1175 (strong), 1116 (medium), 1128 (medium), 975 (weak), 935 (medium), 860 (weak), 823 (medium), 770 (weak), 680 (medium) cm-1.
1H NMR (DMSO-d6): δ 3.85 (3H, s), 6.38 (1H, d, J = 16 Hz), 6.94-6.99 (2H, m), 7.55 (1H, d, J = 16 Hz), 7.62-7.66 (2H, m), 12.2 (1H, br, D2O exchangeable).
Reactions of synthesis:
- Yeild 78-84%. [Ref.1]
CH3OC6H4CHO + CH2(COOH)2 → CH3OC6H4CH=CHCOOH + H2O + CO2
- Yeild 20-35%. [Ref.1]
CH3OC6H4CHO + (CH3CO)2O → CH3OC6H4CH=CHCOOH + CH3COOH
Dissociation:
pKa (1) = 4.54 (25°C, water)
References:
- Dictionary of organic compounds. - Vol. 2, Eccaine - Myrtillin chloride. - London, 1946. - pp. 600
- Seidell A. Solubilities of organic compounds. - 3ed., vol.2. - New York: D. Van Nostrand Company, 1941. - pp. 667
- Свойства органических соединений: Справочник. - Под ред. Потехина А.А. - Л.: Химия, 1984. - pp. 332-333 [Russian]
- Справочник химика. - Т. 3. - М.-Л.: Химия, 1965. - pp. 91 [Russian]
Your requests if no data into database
© Collected Ruslan Anatolievich Kiper, burewestnik@mail.ru

