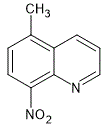

The Richter and Smith procedure for the Skraup synthesis was applied to 207 g. of 3-amino-4-nitrotoluene.
[Two hundred and seven grams of the 3-amino-4-nitrotoluene, 500.7 g of glycerol (dried by passing dry air through the liquid for three hours at 170—180°) and 234.7 g of arsenic pentoxide were weighed into a three-necked flask. This was then equipped with a glycerol-sealed stirrer, thermometer dipping into the liquid and a Pyrex water-cooled condenser with a calcium chloride tube for eliminating atmospheric moisture.
Two hundred and seventy five grams of sulfuric acid (sp. gr. 1.84) was added through the condenser with vigorous stirring, and at such a rate as to maintain the temperature below 130°. The temperature was then held at 130—135° for one hour and finally the mixture was kept in active reflux for six additional hours.]
The reaction mixture was then diluted, cooled and filtered. Additional solid material was precipitated from the liquors by ammonia and was removed by filtration, washed with water and redissolved in dilute hydrochloric acid. This solution was diazotized over a period of three hours by the addition of a large excess of sodium nitrite. The mixture was heated to 80°, cooled to 0° and filtered. The filtrate was made strongly basic with sodium hydroxide, and the resulting precipitate was washed with water and thoroughly air-dried. This crude material was extracted with boiling benzene and the boiling filtrate was treated with charcoal. The product which crystallized on cooling was removed and the remainder recovered by evaporation of the solvent. After two recrystallizations from alcohol 56 g (22%) of pale tan plates, m. p. 136.5—137.5°, were obtained. Manske and co-workers reported the same yield, with m. p. 135°.
© Collected Ruslan Anatolievich Kiper, burewestnik@mail.ru