Main page (Russian)
 Search in database (English)
Search in database (English)
Properties of substance:
ninhydrin oxime
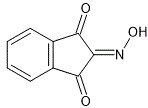
skc-fileGroup of substances:
organic
Empirical formula (Hill's system for organic substances):
C9H5NO3Synthesis 1:
Reference: Teeters, W. O., & Shriner, R. L. A New Preparation of Ninhydrin / Journal of the American Chemical Society. - 1933. - Vol. 55, No. 7 pp. 3028 [doi: 10.1021/ja01334a072]
In a 400-cc. beaker equipped with a stirrer and ice-bath was placed 5 g. of 1,3-indandione dissolved in 200 cc. of dilute sodium hydroxide. After the addition of 2.5 g. of sodium nitrite the mixture was cooled to 0°. Hydrochloric acid was added dropwise until the solution was distinctly acid whereupon the ice-bath was removed and the mixture stirred for thirty minutes at room temperature. The solution was filtered and 5.9 g. (100% of the theoretical) of yellow amorphous powder was obtained. The oxime was recrystallized from acetic acid and formed yellowish green plates; m. p. 200—201° (decomp.).
Anal. Calcd. for C9H5NO3: N, 8.00. Found: N, 7.91 (Dumas).
Synthesis 2:
Reference: Патент США US3,419,616 (от 31.12.1968) Preparation of ninhydrin
A slurry comprising 4.5 grams of 1,3-indandione and 15 ml. of water containing 2.0 grams of concentrated sulfuric acid was stirred at 25 C. for 30 minutes while a solution comprising 2.1 grams of sodium nitrite in 20 ml. of water was added dropwise. The slurry gradually turned from a buff color to yellow. After 40 minutes, the yellow solid was collected by filtration and washed with 10 to 20 ml. water. Drying the yellow solid at 50°C. and 30 mm. Hg vacuum for 8 hours gave 5.05 grams (93.5% yield) of 2-oximino-1,3-indandione having a melting point of 204—208°C.References:
Your requests if no data into database
© Collected Ruslan Anatolievich Kiper, burewestnik@mail.ru



