Main page (Russian)
 Search in database (English)
Search in database (English)
Properties of substance:
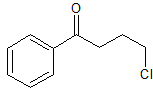
3-chloropropyl phenyl ketone
skc-fileSynonyms:
4-chloro-1-phenylbutan-1-one
Group of substances:
organic
Empirical formula (Hill's system for organic substances):
C10H11ClOStructural formula as text:
C6H5COCH2CH2CH2ClMolar/atomic mass: 182.647
Melting point (°C):
19-20CAS ¹: 939-52-6
Solubility (g/100 g of solvent):
diethyl ether: very soluble [Ref.]
ethanol: very soluble [Ref.]
petroleum ether: sparingly soluble [Ref.]
water: insoluble [Ref.]
Synthesis 1:
Reference: Conant, J. B., Segur, J. B., & Kirner, W. R. Gamma-chloropropyl-phenylketone / Journal of the American Chemical Society. - 1924. - Vol. 46, No. 8 pp. 1884 [doi: 10.1021/ja01673a015]
C6H5MgBr + NC(CH2)3Cl → C6H5C(=NMgBr)(CH2)3Cl
C6H5C(=NMgBr)(CH2)3Cl + HCl → C6H5C(=NH)(CH2)3Cl + MgBrCl
C6H5C(=NH)(CH2)3Cl + H2O + HCl → C6H5CO(CH2)3Cl + NH4Cl
Thirteen g. of γ-chlorobutyronitrile in 100 cc. of dry ether was slowly added to an ethereal solution of phenylmagnesium bromide prepared from 5.2 g. of magnesium and 40 g. of bromobenzene; the reaction mixture was kept cold in an ice-bath. After two to four hours the mixture had become a pasty mass and was poured into a mixture of ice and hydrochloric acid and immediately extracted with ether. This extraction removed any unchanged bromobenzene, benzene or diphenyl, and the first product of the reaction (which was the imide, C6H5C(=NH)CH2CH2CH2Cl) stayed in the aqueous solution as the hydrochloride. This imide was only slowly hydrolyzed to the ketone in the ice-cold solution, but on allowing the solution to come to room temperature and to stand for 24 hours the hydrolysis was complete and the ketone separated as a dark brown oil. This was dissolved in ether, dried and distilled under diminished pressure; 12 g. of a light yellow oil was collected between 135° and 150° at 11 mm. (most of this boiled at 133.5—135°); yield, 54%. It was difficult to purify the compound completely by fractional distillation but it could be obtained very pure by recrystallizing it from anhydrous petroleum ether at -20°. It separated from a fairly dilute solution in the form of glistening white rosettes. These were filtered off with suction on a funnel surrounded with ice and dried in a vacuum desiccator over paraffin and sulfuric acid; m. p., 19—20°.
Anal. Calc. for C10H11OCl: Cl, 19.42. Found: 19.39.
The compound is insoluble in water, slightly soluble in petroleum ether and readily soluble in alcohol and ether; it has no lachrymatory properties, in contrast to α-chloroketones.
Vapour pressure (Torr):
11 (133.5°C)
References:
- Journal of the American Chemical Society. - 1924. - Vol. 46, No. 8. - pp. 1884 [DOI: 10.1021/ja01673a015]
Your requests if no data into database
© Collected Ruslan Anatolievich Kiper, burewestnik@mail.ru

