
A 5-liter round-bottom Pyrex flask, with one additional neck supplied, is fitted with a mercury-sealed all-metal stirring device and pulley, conveniently made by using a ball-bearing bicycle wheel hub as support. The stirrer shaft is allowed to extend for 3—4 in. through the rubber stopper closing the large opening of the flask, and a stirrer made of glass tubing is slipped over the lower portion of the drive shaft to which it is made fast, using a section of heavy-walled rubber tubing to make the attachment and protect the contents of the flask from action with metal. The design of the stirrer should be such that at 200—300 R.P.M. the contents of the flask are kept at a condition of turbulent rotary agitation. The flask is supported above an adjustable electric hot plate to provide adequate temperature control. A reflux condenser and thermometer are then attached to the second neck of the reaction flask.
Two pounds of crude o-nitroaniline and 2 pounds and 1 ounce of arsenic pentoxide are introduced into the flask; 4 pounds and 6.5 ounces of anhydrous glycerol are added. While stirring, 4 pounds of concentrated sulfuric acid is added gradually through the reflux condenser, in such a manner that the temperature of the reacting mixture does not exceed 110°C. After 1 hour of continuous stirring and gradual elevation of the temperature to 120°C., the stirring and elevating of the temperature is continued at the rate of 5°C. per hour until the temperature reaches 135°C. Care should be used in raising the temperature to 140°C. to prevent the reaction advancing too rapidly. Finally after 6 hours heating the reflux condenser begins to return appreciable quantities of reactants to the flask, and the amount of reflux gradually diminishes during the last 2 hours heating, as the temperature of the reaction gradually falls even while a fixed amount of heat is being applied. Finally after seven to eight hours the refluxing liquid is colorless and the reaction is complete.
The cold contents of the reaction flask are poured into a 20-gallon acid resisting, glazed earthenware crock to which has been added 30—40 pounds of finely chopped ice. The mixture is stirred by the use of an efficient, enameled metal, inverted, Y-shaped stirrer or substitute, saddled to the top of the earthenware container and driven by motor to provide turbulent stirring. Three and one-half pounds of caustic soda dissolved in 3 gallons of water are now added through a small-bore siphon delivery tube during a period of approximately two hours. The reaction mixture should at all times be chilled to 0°C. or lower, and additional chipped ice added as necessary. After the solution has been neutralized as tested, using litmus paper, and a slight excess of alkali has been added, the crude 8-nitroquinoline in the form of a yellowish brown precipitate is filtered, using a 14-in. Buchner funnel. The crude product precipitated from a solution of such dilution (approximately 20 gallons) requires little or no washing to be obtained substantially free from sodium salts. The final product need not be dried before reduction to 8-aminoquinoline in the next step, and since it need not be further purified, the yield in the first two steps together is to be considered subsequently.
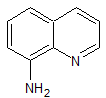
Reduction of 8-nitroquinoline forming 8-aminoquinoline
For working up moderately large batches of material a 12-quart, cake-mixing machine of the Hobart type is used, having a rotating and planetary motion batter-beating type of stirrer. Twelve pounds of powdered iron is mixed with just enough water to moisten the mix thoroughly, and 2.5 pounds of commercial hydrochloric acid added. To the hot mixture thus obtained the moist 8-nitroquinoline is added in small portions to maintain approximately the same temperature, adding water from time to time to replace that lost by evaporation. The iron and acid added are sufficient for the reduction of the 8-nitroquinoline from two of the first Skraup syntheses. After all the nitro compound has been added, while keeping the reaction mixture in the form of a thick paste, the reaction vessel is heated and stirred for an additional 2 hours. Finally sufficient flake caustic soda is added with stirring to neutralize the 8-aminoquinoline hydrochloride and to form sufficient iron precipitate to thicken materially the pasty reaction mixture. The aminoquinoline is then extracted, using several portions of benzene totalling 10—15 liters, and using the same mixing machine with intermediate filtration of the insoluble matter by reduced pressure in a 14-in. Buchner funnel. The benzene solution is finally concentrated by distillation at atmospheric pressure in a 5-liter, vacuum distilling, Claisen-type still until the boiling temperature reaches 100°C. Finally the 8-aminoquinoline is distilled under 25 mm. pressure, at which it boils at 175°C. A capillary air vent is used in the usual fashion to prevent bumping and to regulate pressure. The yield for the two steps thus described is approximately 50 per cent of theoretical, based upon the o-nitroaniline.
© Collected Ruslan Anatolievich Kiper, burewestnik@mail.ru