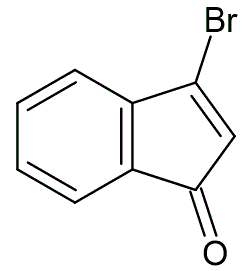

To a solution of 1-indanone (0.5 g, 3 mmol) in carbon tetrachloride (20 mL), at 25 °C, were added dry recrystallized N-bromosuccinimide (1.1 g, 63 mmol), and azobisisobutyronitrile (11 mg). The mixture was heated at reflux and illuminated with a 125W light. After one hour the reaction was cooled to 5 °C, and triethylamine (1.52 mL, 10.5 mmol) was added. The reaction mixture was allowed to stir for one hour at 25 °C. The resulting solids were removed by filtration, and washed with carbon tetrachloride. The filtrate was concentrated under reduced pressure and diluted with diethyl ether (70 mL). The organic portion was washed with 0.1 N HCl (50 mL) and the aqueous layer extracted with diethyl ether (25 mL). The organic layers were combined, dried over Na2SO4, filtered, then concentrated under reduced pressure to afford pure 3-bromo-1-indenone (0.72 g, 99% yield) as an orange solid, mp 57—58.5 °C.
Rf 0.49 petroleum ether : ethyl acetate (90 : 10).
HRMS calcd. for C9H5BrO (M+): 207.952. Found: 207.953.
IR (CHCl3): 1710 (strong), 1605 (medium), 1540 (medium), 1455 (medium), 1365 (medium), 1300 (weak), 1280 (weak), 1240 (strong), 1180 (medium), 1140 (medium), 1075 (medium), 1045 (medium), 1010 (medium), 945 (weak), 915 (medium), 885 (weak), 830 (medium) cm-1.
1H NMR (CDCl3): δ 6.21 (1H, s), 7.18-7.50 (4H, m).
© Collected Ruslan Anatolievich Kiper, burewestnik@mail.ru